

So, at pH 9.5, the ratio of the positively charged amino group to a neutral amino group is 100:1 (at the pKb it would be 50:50). Same is true for the amino groups (pKb is at pH 11.5 for arginine). The carboxyl group is negatively charged in a solution much higher than the pKa of the carboxyl group (which is pH 2.48 for arginine). You are not correct that the groups are truly neutral. On the other hand, if amino acids have two carboxyl groups and only one amino group (like glutamic acid and aspartic acid), they are negatively charged at pH7 (- vs. It does not store any personal data.If an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are positively charged (++ vs. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. The cookies is used to store the user consent for the cookies in the category "Necessary". The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics".
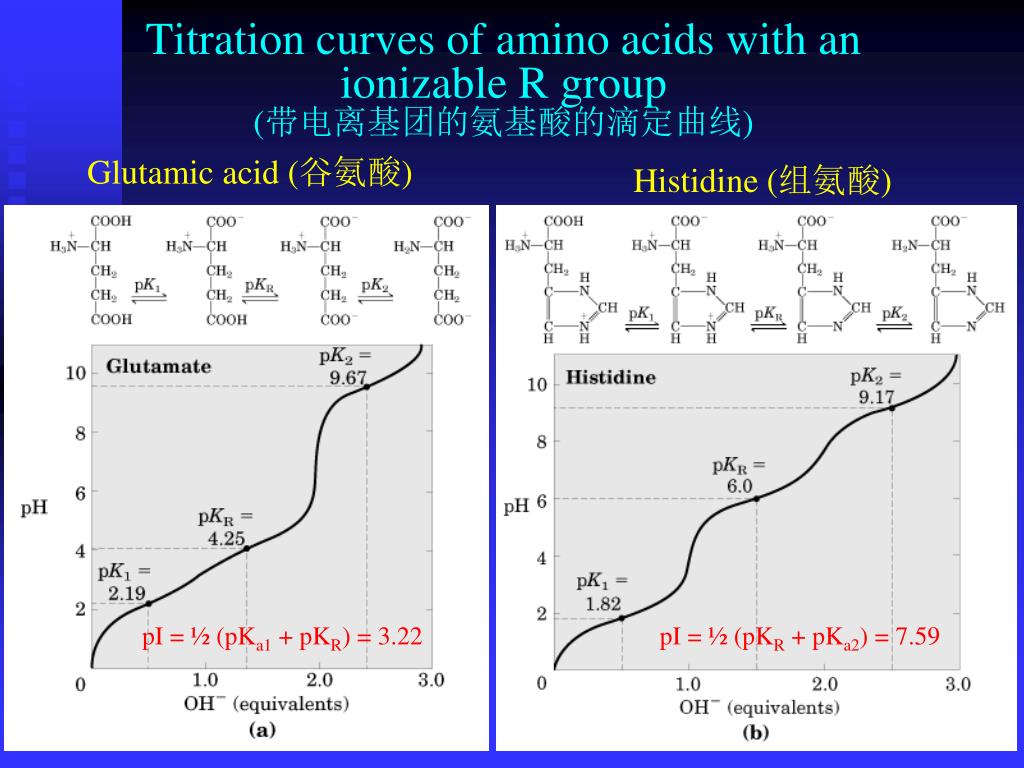
These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. This can then be combined with the original volume of the analyte solution to determine its concentration. How do you find equivalence point from concentration?Īt the equivalence point the moles of added base will be equal to the moles of original acid, this allows the determination of the number of moles of original acid. The is the case of the neutralization of weak acid/base with strong base/acid. The pH at the equivalence point is not equal to 7 when the salt formed in the neutralization is either acidic or alkaline instead of neutral salt. These gels won’t have the same resolution as CE. Isoelectric poit of protein can be measured with capillary electrophoresis in the mode of IEF (isoelectric focusing). How are isoelectric point pI determined experimentally? How do you calculate the pI of a Tetrapeptide? Note: The isoelectric point is given by the average of the pKa values that involve the zwitterions, not just by the pKa values that describe the carboxylic acid group and the amine group. Now, for lysine, the pKa1 is equal to 2.18, pKa2 is equal to 8.95 and pKa3 is equal to 10.53. How do you calculate the isoelectric point of lysine? The isoelectric zone of glycine extends from approximately pH 4.5 to pH 7.5, and its isoelectric point is at pit 6.082 (6). What is the isoelectric point of glycine? At solution pH that is above the pI, the surface of the protein is predominantly negatively charged, and therefore like-charged molecules will exhibit repulsive forces.

The isoelectric point (pI) is the pH of a solution at which the net charge of a protein becomes zero. At what point does the isoelectric point or pI occur? Thus, the pH value may require adjustment when the isoelectric point is chosen after the addition of metal ions to the solution. For example, the isoelectric point of insulin is 5.3, but it increases to 6.2 when combined with Zn2+. The isoelectric point of a protein will shift if the protein combined with metal ions. How do you find the charge when given the pH and pI? Calculate the pKa with the formula pKa = -log(Ka).


 0 kommentar(er)
0 kommentar(er)
